what is the most effective way to split hydrogen from water
With hydrogen power stations in California, a new Japanese consumer car and portable hydrogen fuel cells for electronics, hydrogen every bit a zero emission fuel source is now finally becoming a reality for the boilerplate consumer. When combined with oxygen in the presence of a catalyst, hydrogen releases free energy and bonds with the oxygen to form water.
The two chief difficulties preventing united states of america from having hydrogen ability everything we have are storage and production. At the moment, hydrogen production is free energy-intensive and expensive. Unremarkably, industrial production of hydrogen requires high temperatures, big facilities and an enormous amount of energy. In fact, it usually comes from fossil fuels similar natural gas – and therefore isn't really a nil-emission fuel source. Making the procedure cheaper, efficient and sustainable would go a long way toward making hydrogen a more commonly used fuel.
An excellent – and abundant – source of hydrogen is h2o. But chemically, that requires reversing the reaction in which hydrogen releases energy when combining with other chemicals. That means we have to put free energy into a compound, to get the hydrogen out. Maximizing the efficiency of this process would be significant progress toward a make clean-energy future.
One method involves mixing water with a helpful chemic, a catalyst, to reduce the amount of energy needed to break the connections between hydrogen and oxygen atoms. At that place are several promising catalysts for hydrogen generation, including molybdenum sulfide, graphene and cadmium sulfate. My enquiry focuses on modifying the molecular backdrop of molybdenum sulfide to make the reaction even more than effective and more efficient.
Making hydrogen
Hydrogen is the virtually abundant element in the universe, simply information technology's rarely bachelor equally pure hydrogen. Rather, it combines with other elements to grade a keen many chemicals and compounds, such as organic solvents like methanol, and proteins in the human trunk. Its pure form, H₂, tin can used as a transportable and efficient fuel.
There are several ways to produce hydrogen to be usable every bit fuel. Electrolysis uses electricity to split water into hydrogen and oxygen. Steam methane reforming starts with marsh gas (four hydrogen atoms bound to a carbon cantlet) and heats it, separating the hydrogen from the carbon. This energy-intensive method is usually how industries produce hydrogen that is used in things like producing ammonia or the refining of oil.
The method I'k focusing on is photocatalytic water splitting. With a goad's help, the amount of energy needed to "split" h2o into hydrogen and oxygen can be provided by some other abundant resource – light. When exposed to light, a proper mixture of water and a catalyst produces both oxygen and hydrogen. This is very attractive to manufacture considering information technology and then allows united states to employ h2o as the source of hydrogen instead of muddy fossil fuels.
Understanding catalysts
Just as not every two people outset upwards a conversation if they're in the same elevator, some chemic interactions don't occur only considering the 2 materials are introduced. H2o molecules tin can be split into hydrogen and oxygen with the improver of energy, only the amount of energy needed would be more than would exist generated equally a result of the reaction.
Sometimes information technology takes a third party to get things going. In chemistry, that's called a catalyst. Chemically speaking, a goad lowers the amount of energy needed for 2 compounds to react. Some catalysts function but when exposed to light. These compounds, like titanium dioxide, are called photocatalysts.
With a photocatalyst in the mix, the free energy needed to split water drops significantly, and then that the effort nets an free energy gain at the end of the process. We can make the splitting even more efficient by adding some other substance, in a role called co-catalyst. Co-catalysts in hydrogen generation change the electronic structure of the reaction, making it more than constructive at producing hydrogen.
So far, there aren't any commercialized systems for producing hydrogen this mode. This is in part because of cost. The best catalysts and co-catalysts nosotros've constitute are efficient at helping with the chemical reaction, but are very expensive. For instance, the first promising combination, titanium dioxide and platinum, was discovered in 1972. Platinum, however, is a very expensive metallic (well over US$1,000 per ounce). Even rhenium, another useful catalyst, costs around $seventy an ounce. Metals like these are so rare in the Earth's crust that this makes them not suitable for large-scale applications fifty-fifty though there are processes being adult to recycle these materials.
Finding a new catalyst
There are many requirements for a good catalyst, such every bit beingness able to exist recycled and being able to withstand the heat and pressure level involved in the reaction. But just as crucial is how mutual the material is, because the most abundant catalysts are the cheapest.
1 of the newest and most promising materials is molybdenum sulfide, MoS₂. Because it is fabricated upwards of the elements molybdenum and sulfur – both relatively common on Earth – it is far cheaper than more traditional catalysts, well under a dollar per ounce. It likewise has the correct electronic properties and other attributes.

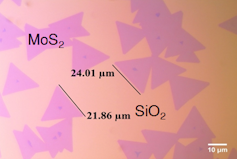
Before the late 1990s, researchers had found that molybdenum sulfide was non particularly constructive at turning h2o into hydrogen. Just that was because researchers were using thick chunks of the mineral, essentially the course it's in when mined from the ground. Today, however, we can use processes like chemical vapor deposition or solution-based processes to create much thinner crystals of MoS₂ – even down to the thickness of a unmarried molecule – which are vastly more efficient at extracting hydrogen from water.
Making the process even better
Molybdenum sulfide can exist fabricated even more than effective by manipulating its physical and electrical properties. A process known every bit "phase change" makes more of the substance available to participate in the hydrogen-producing reaction.
When molybdenum sulfide forms crystals, the atoms and molecules on the outside of the solid mass are ready to accept or donate electrons to water when excited by light to drive the creation of hydrogen. Normally, the MoS₂ molecules on the inside of the construction will not donate or have electrons as efficiently as the edge sites, and so tin't aid as much with the reaction.
Only adding energy to the MoS₂ by bombarding it with electrons, or increasing the surrounding pressure, causes what is called "stage modify" to occur. This phase modify is non what you learn in basic chemistry (involving one substance taking forms of gas, liquid or solid) only rather a slight structural change in the molecular arrangement that changes the MoS₂ from a semiconductor to a metal.
Every bit a result, the electrical properties of the molecules on the inside go available to the reaction as well. This makes the same amount of goad potentially 600 times more constructive in the hydrogen evolution reaction.
If the methods behind this sort of quantum can be perfected, then we may be a big footstep closer to making hydrogen production cheaper and more efficient, which in turn will move u.s.a. toward a hereafter powered past truly clean, renewable energy.
Source: https://theconversation.com/finding-better-ways-to-get-hydrogen-fuel-from-water-62592
0 Response to "what is the most effective way to split hydrogen from water"
Post a Comment